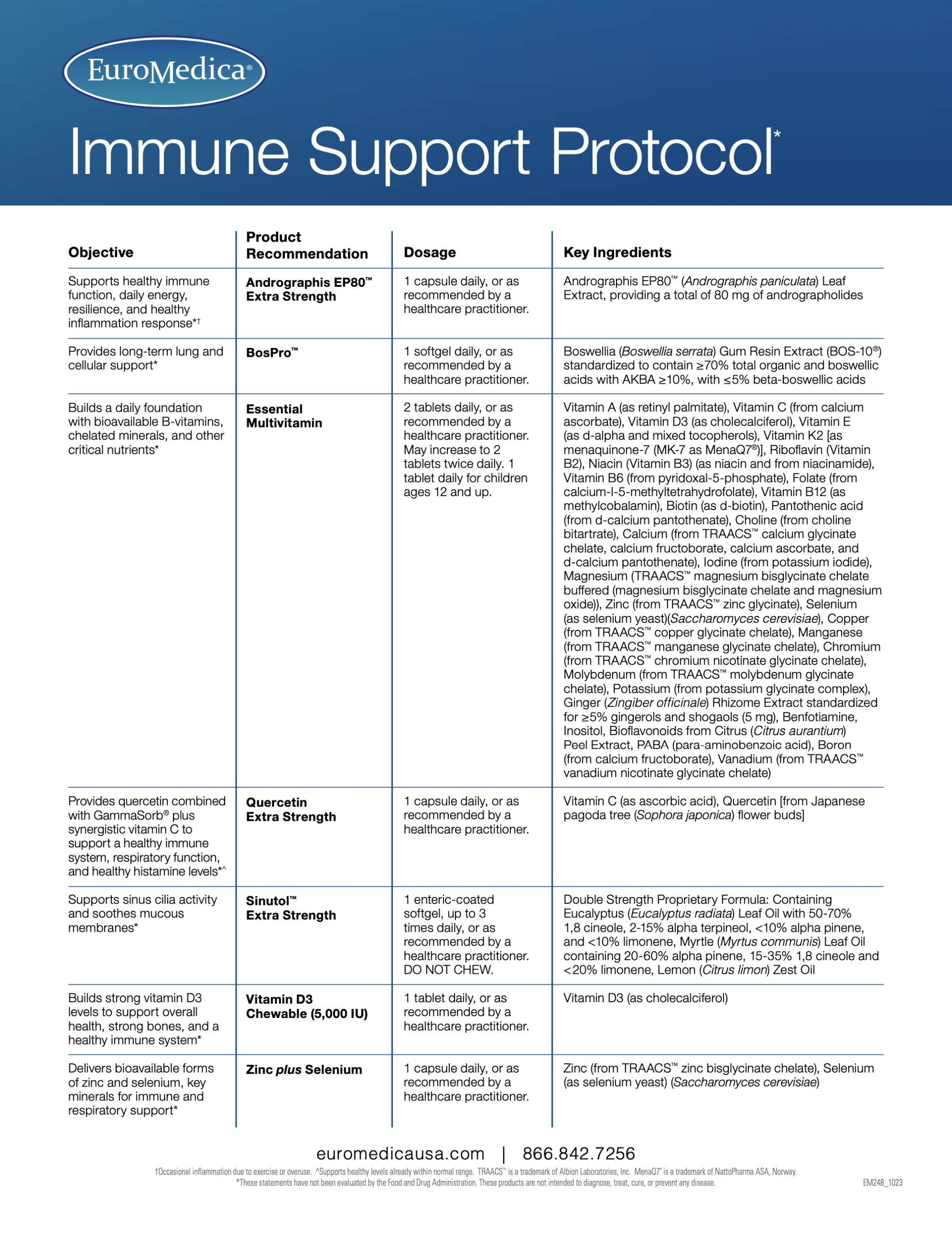
Sinutol™ Extra Strength
Enteric Coated – Supports Sinus Cavities and Soothes Mucous Membranes*
Sinutol Extra Strength provides the soothing power of eucalyptus, myrtle, and lemon extract for sinus and immune support. Sinutol Extra Strength supports healthy sinus cilia activity. Combined in an enteric-coated softgel to eliminate burping or aftertaste, Sinutol Extra Strength helps provide support without drowsiness or jitters.*
Sinus, Bronchial, and Lung Support!*
- Refreshing Eucalyptus (Eucalyptus radiata) provides powerful compounds, including alpha pinene, and 1,8 cineole. It has long been studied and shown to support healthy, clear sinus passages.*
- Strong Myrtle (Myrtus communis) supports a healthy respiratory environment and, like eucalyptus, also provides alpha pinene and 1,8 cineole.*
- Powerful Lemon (Citrus limon) provides additional sinus and immune support.*
Serving Size: 1 Enteric-Coated Softgel
Servings Per Container: 30
Ingredient
Amount/Serving
% Daily Value
Double Strength Proprietary Formula
325 mg
**
Containing Eucalyptus (Eucalyptus radiata) Leaf Oil with 50-70% 1,8 cineole, 2-15% alpha terpineol, < 10% alpha pinene, and < 10% limonene, Myrtle (Myrtus communis) Leaf Oil containing 20-60% alpha pinene, 15-35% 1,8 cineole and < 20% limonene, Lemon (Citrus limon) Zest Oil
**Daily Value not established
Other Ingredients: extra virgin olive oil, gelatin, glycerol, water, enteric coating (purified water, ethylcellulose, sodium alginate, ammonium hydroxide, medium chain triglycerides, oleic acid, vegetable source stearic acid).
No: sugar, salt, yeast, wheat, gluten, corn, soy, dairy products, artificial coloring, artificial flavoring, or artificial preservatives.
Recommendations: 1 enteric-coated softgel, up to 3 times daily, or as recommended by your healthcare practitioner. DO NOT CHEW.
If pregnant or nursing, consult a healthcare practitioner before use.