Tri-Iodine®
Supports Thyroid Function, Cellular Defense^, and Hormone Balance*†
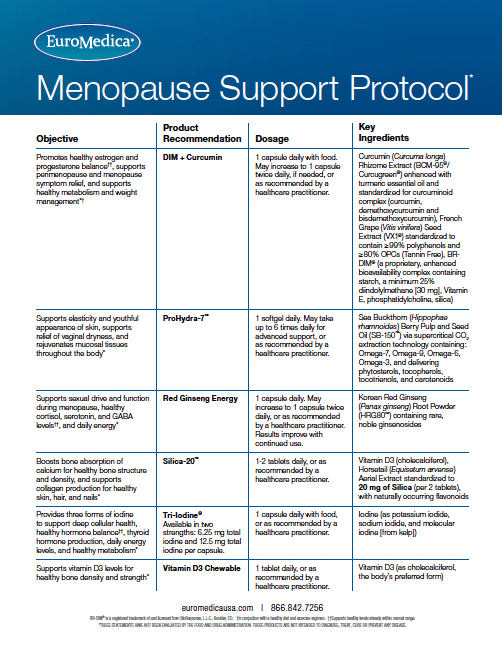
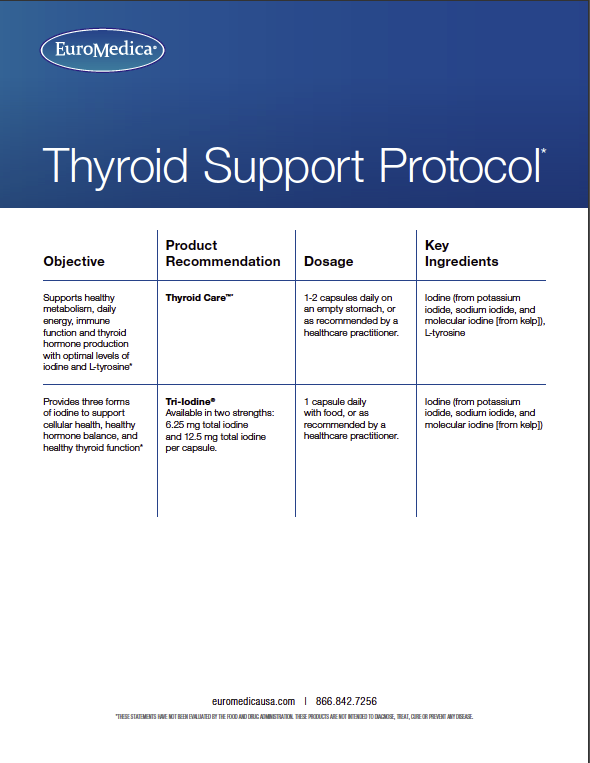
Tri-Iodine provides three beneficial forms of iodine, an essential element. Available in two strengths for targeted supplementation: 6.25 mg total iodine (equivalent to 6,250 mcg), and 12.5 mg total iodine (equivalent to 12,500 mcg) per capsule. Optimal levels of iodine are imperative to many systems in the body including:
- Thyroid
- Breast
- Brain
- Immune
- Prostate
- Metabolic Function*
^Protection from oxidative stress and damage
†Supports healthy levels already within normal range
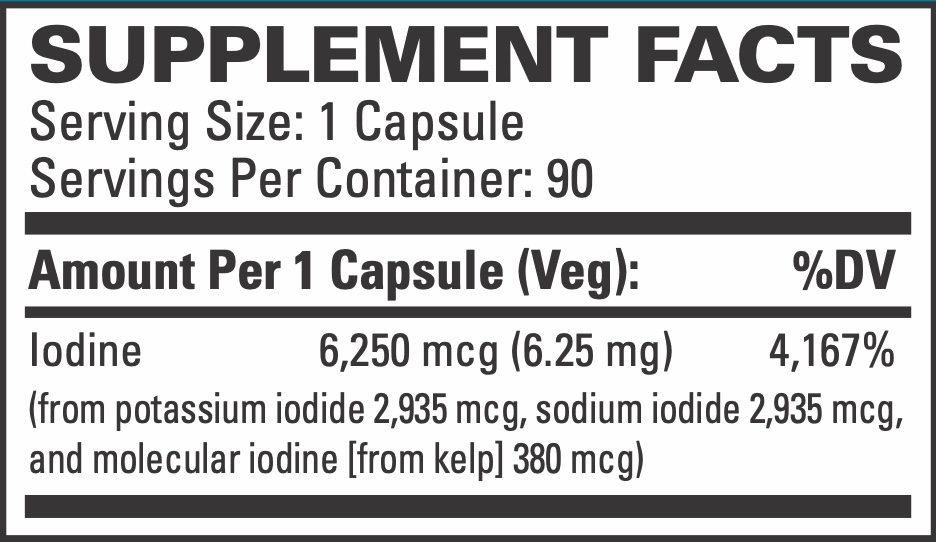
Serving Size: 1 Capsule
Servings Per Container: 90
Ingredient
Amount/Serving
% Daily Value
Iodine
(from potassium iodide 2,935 mcg, sodium iodide 2,935 mcg, and molecular iodine [from kelp] 380 mcg)
6,250 mcg (6.25 mg)
4,167%
OR
Iodine
(as potassium iodide 6,060 mcg, sodium iodide 6,060 mcg, and molecular iodine [from kelp] 380 mcg)
12,500 mcg (12.5 mg)
8,333%
**Daily Value not established
Other Ingredients: microcrystalline cellulose, hydroxypropyl methylcellulose (vegetable cellulose capsule), silica.
No: sugar, salt, yeast, wheat, gluten, corn, soy, dairy products, artificial coloring, artificial flavoring, or artificial preservatives.
Recommendations: 1 capsule daily with food, or as directed by your healthcare practitioner.
If trying to conceive, pregnant, or nursing, consult a healthcare practitioner before use.