Clinical Glutathione™
Patented, Reduced Oral Glutathione for Vital Support*
Clinical Glutathione featuring Sublinthion® —A First of Its Kind
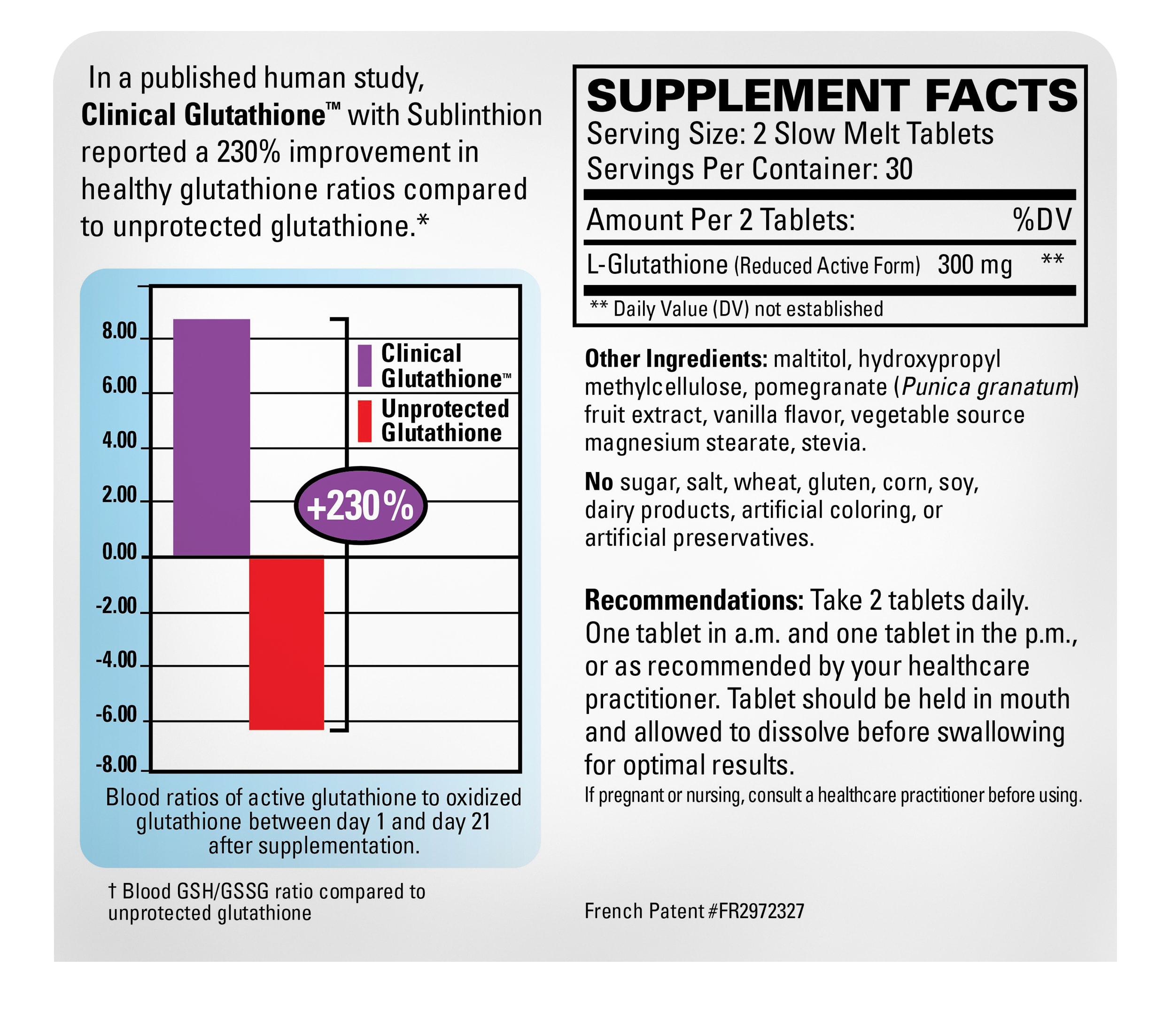
Sublinthion is a unique, protected glutathione from France produced by a patented process. In a published human study, Clinical Glutathione with Sublinthion improved the reduced glutathione vs. oxidized glutathione blood ratios in the body by 230%.*†
Clinical Glutathione is specially formulated as a slow-melt tablet, protected by a unique, patented process. It supports beneficial levels of active glutathione in the bloodstream and improves the ratio of active glutathione to oxidized glutathione in a way that other approaches can’t.*
Clinical Glutathione:
- Improves blood ratios by 230%*†
- Slow melt tablet for optimal benefits
- Clinically tested, European innovation
- Exclusive, patented, protected delivery system
†Blood GSH/GSSG ratio compared to unprotected glutathione
Serving Size: 2 Slow Melt Tablets
Servings Per Container: 30
Ingredient
Amount/Serving
% Daily Value
L-Glutathione (reduced active form)
300 mg
**
** Daily Value not established
Other Ingredients: maltitol, hydroxypropyl methylcellulose, pomegranate (Punica granatum) fruit extract, vanilla flavor, vegetable source magnesium stearate, stevia.
No: sugar, salt, wheat, gluten, corn, soy, dairy products, artificial coloring, or artificial preservatives.
Recommendations: Take 2 tablets daily. One tablet in a.m. and one tablet in the p.m., or as recommended by your healthcare practitioner. Tablet should be held in mouth and allowed to dissolve before swallowing for optimal results.
If pregnant or nursing, consult a healthcare practitioner before use.